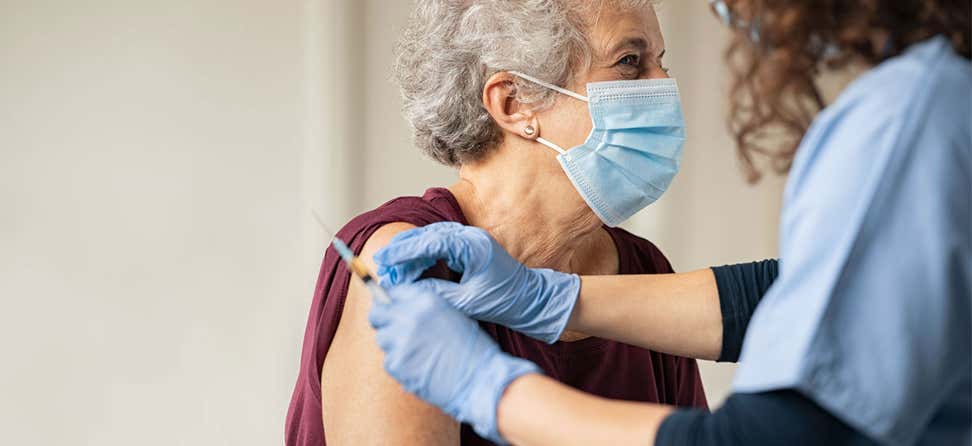
The development of effective vaccines has been essential in our collective fight against COVID-19. These vaccines play a key role in reducing the severity of illness, hospitalizations, and deaths, especially among people age 65 and older.
“We have more tools than ever to prevent the worst outcomes from COVID-19,” said CDC Director Mandy Cohen, MD, MPH, in a news release last September.
As we navigate this phase of the pandemic, getting vaccinated is still the best defense against hospitalization and death related to COVID-19. Older adults continue to be at higher risk of complications due to age. As we head into cold and flu season, staying up to date with recommended vaccinations can help boost your immunity and give you the most protection possible.
If you’re wondering which COVID vaccine is best for seniors, see below for common questions and answers on the latest vaccine guidance.
What COVID-19 vaccines are currently available?
The COVID-19 vaccines available and recommended in the U.S. include Pfizer-BioNTech, Moderna, and Novavax.
In September 2023, the 2023–2024 updated Pfizer-BioNTech and Moderna COVID-19 vaccines were recommended by CDC for use in the U.S. The updated Novavax COVID-19 vaccine was approved by the FDA in early October. These updated vaccines more closely target the XBB lineage of the Omicron variant, which is now widely circulating.
What about the COVID vaccine for 65 and older?
As of February 2024, the the Centers for Disease Control and Prevention (CDC) recommend that everyone age 65 and older receive an extra dose of the 2023-2024 updated COVID-19 vaccine for added protection that may have waned over time. This extra dose is given in addition to the one dose that is currently recommended for everyone age 5 years and older. You should wait at least four months after getting the last dose of any COVID-19 vaccine to get a new dose.
If you're 65 and older, you are considered up to date when you receive two updated COVID vaccine doses.
If you're an older adult who is moderately or severely immunocompromised, you may get an additional dose of an updated COVID-19 vaccine. Talk to your health care provider about the appropriate vaccine schedule for you.
If you're not able to (or choose not to) get an updated Pfizer-BioNTech or Moderna COVID-19 vaccine, you may be able to get a Novavax booster. To do so, you must:
- Be 18 or older
- Have completed a COVID vaccine primary series at least 6 months ago
- Have not received any other COVID booster dose
How long does protection from the COVID vaccine last?
Your immunity to COVID-19 should improve within about two weeks after getting the vaccine. While this won't provide 100% protection from getting the virus, it should help reduce the severity and duration of symptoms if you do become ill. Boosted immunity from the COVID vaccine typically lasts for a few months.
Are the COVID-19 vaccines safe for older adults?
Regardless of which vaccine you get, you can be assured of its safety. These vaccines were all evaluated using the same rigorous process as all other vaccines approved by the FDA. Drug companies were required to provide extensive safety data from clinical trials involving tens of thousands of people.
You may experience temporary, minor side effects with the vaccine, which are normal signs your body is building protection against the virus. Common reported side effects include:
- Pain and swelling at the injection site or upper arm
- Muscle aches and weakness
- Fever and/or chills
- Tiredness
- Headache
When you receive your vaccine, you will be asked to wait at least 15-30 minutes to check for an allergic reaction. This kind of reaction is rare, but may occur in some people, especially those with a history of anaphylaxis. If you fall into this category, speak with your doctor before getting the vaccine.
What if I recently had COVID?
If you recently had a COVID-19 infection, you may consider waiting up to three months before you get another vaccine. This is because you develop some immunity to the virus after getting sick, and reinfection is not likely to occur within weeks (or even months) after infection. But if you or someone close to you is at high risk for severe illness, you should not delay getting a booster for too long. Talk to your doctor about timing.
Is the COVID vaccine free?
Free COVID-19 vaccines are available to all people age 6 months and older living in the U.S., regardless of insurance or immigration status.1 Getting vaccinated will cost you nothing, whether you have Medicare, Medicaid, private insurance, or no insurance at all. You should still bring your insurance card with you to your vaccine appointment, since vaccine providers can bill insurance companies for the cost.
Where can I get the COVID vaccine?
To find a COVID vaccine appointment near you:
- Call your local health department. Many communities have free vaccine clinics at senior centers and other locations. You may be required to register online and schedule a time.
- Visit Vaccines.gov. This website makes it easy to find a COVID-19 or flu vaccination site near you. You can also call 1-800-232-0233 (TTY 888-720-7489).
- Check with your local pharmacy. While some pharmacies offer walk-in vaccinations, others require you to schedule an appointment on their website. Your pharmacist can answer any questions you have about the updated COVID vaccine.
- Check with your doctor, health clinic, or hospital. They likely also have COVID vaccines on site. If not, your doctor should be able to recommend an alternative location.
- Reach out to your area agency on aging or senior center. To find yours, contact Eldercare Locator (1-800-677-1116).
If you need help with getting the COVID-19 vaccine, please don’t hesitate to ask family, friends, or neighbors.
Can I get my COVID booster and flu shot at the same time?
It’s ok to receive your COVID and flu vaccines during the same visit. However, if you’ve experienced soreness at the injection site or other side effects in the past, you may wish to schedule separate appointments to minimize your discomfort.
Will I still have to wear a mask and take other precautions after I’m vaccinated?
Although the vaccines are very effective at reducing illness, hospitalization, and death, you should still take basic precautions to protect yourself and others from COVID-19. This includes washing your hands frequently and avoiding large crowds. You should also wear a high-quality mask (like an N95 mask) that fits snugly around your nose and mouth if:
- You’re at high risk for severe COVID-19
- You’ve been exposed to COVID-19
- You suspect you have COVID
- You’re in a crowded and/or poorly ventilated space
The bottom line
For older adults—especially those with chronic conditions like heart disease and diabetes—the COVID-19 vaccine can prevent severe illness or death from the virus.
“COVID-19 continues to pose a threat to older Americans,” says Josh Hodges, NCOA’s Chief Customer Officer. “Keeping up to date with your vaccines is an easy yet powerful way to protect yourself, your family, and your community.”
This article was updated in February 2024, based on the latest public health information at the time of publication. Read the most up-to-date article from NCOA on COVID and older adults.
Sources
1. U.S. Department of Health and Human Services. COVID-19 Vaccines. Found on the internet at https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html
2. Centers for Disease Control and Prevention. Getting a Flu Vaccine and a COVID-19 Vaccine at the Same Time. Found on the internet at https://www.cdc.gov/flu/prevent/coadministration.htm